Hands-On-Physics | |
Heat & Temperature
|
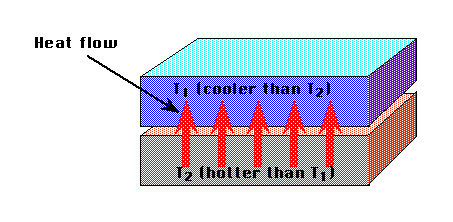 Figure P3
Figure P3A Model of Heat Flow
When two objects are in contact, heat flows from the hotter object to the
cooler. You will find it helpful to have a mental model of heat transfer
when two objects are in contact. Think about the atoms in the hot and cold
substances.

Imagine a bunch of hot atoms in violent motion next to a bunch of cool
atoms that move less. Heat flows because hotter atoms, which have a greater
random motion, hit cooler ones. When they collide, some of the energy of
a hotter atom is transferred to a cooler one, speeding it up. This simplified
model can help you remember the idea, but it is not entirely accurate. Actually,
atoms do not have to collide to exchange thermal energy. Each atom has an
electric and magnetic field around it and these fields can transfer energy.
Metals conduct electricity because they have free electrons that bounce
around like a gas; this electron gas interacts with atoms and can transfer
energy, too.
Factors Influencing Heat Flow
We can develop an equation for the rate of heat transfer. Think about all
the different kinds of factors that might influence the flow of heat. Imagine
a material sandwiched between objects that are hot and cold. The amount
of heat that will flow depends on the thickness, area, the temperature difference,
and the nature of the material.
The effect of all these factors can be summarized in the Heat Flow Equation.
Many things flow. Water flows in ditches and pipes, electric charge flows
through wires, air flows over the wings of an airplane, and heat flows from
hot to cold. The rate a which a flow can fill a container depends of the
speed of the flow the effective cross section of the ditch or pipe or wire.
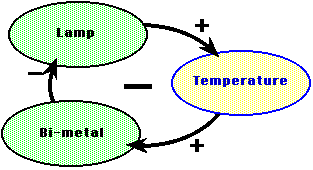
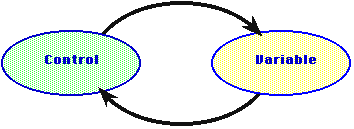
Perhaps more interesting is the principle of negative feedback. In this
control process, a changing condition of some system is regulated to keep
it more or less constant. When the actuator increases the variable reaches
some set maximum, a sensor sends a message to the actuator, and the cause
of the change is shut off or reversed.

Controlling Heat Flow
In many situations it is important to control the transfer of heat. Some
things get too hot unless heat can be removed. Examples include an elephant
in the sun at noon, a car engine, a nuclear plant, and a microprocessor
chip. Each of these need ways of removing heat quickly and easily. In other
cases, you want to preserve heat by reducing heat loss as much as possible.
Examples include a building in the winter, a jug of hot coffee, and a baby
incubator. In these situations, the goal is to reduce heat transfer.
Incubators use general principles which appear in the physical world in
many different forms. Flow and collection are controlled with negative feedback.
In an incubator the flow of heat out (cooling) is compensated for by pouring
new heat in. The inflow of new heat is controlled by sensor with a switch
(thermostat) which turns the inflow off when the temperature is high enough
and on again when the incubator is too cool.