Hands-On-Physics |
|
Heat & Temperature
|
Heat and cold are considerations in much of what we do. The air temperature needs to be about right for us to work and play comfortably. It should be cooler to keep food from spoiling and warmer to cook it. Gasoline engines work by letting hot gas expand and cool; storms are driven by collisions of hot & cold air. The control of temperature and the regulation of heat are important in many aspects of human activity.
You can usually warm something by adding energy. The added energy can be from light, electricity, friction, a chemical reaction, nuclear reaction, or any other kind of energy. When first added to a substance, energy might be concentrated in one atom, but this one will soon bump into others and spread the energy. Eventually, every atom or molecule in the substance will move a bit faster. When the added energy is spread throughout a substance, it is then called heat energy, thermal energy, or, simply heat. All three terms mean the same thing. Heat is a form of energy, so it has the units of energy. In the SI system, this is Joules. Many other units to measure thermal energy are in common use. Calories and BTU's are common heat units.
You cannot measure heat directly, but you can detect its effect on a
substance. Changes in heat can usually be detected as changes in temperature.
Usually, when you add energy to a bunch of atoms they move faster and get
hotter. Similarly, if you remove energy from a bunch of atoms, they usually
move less and get cooler.
Figure P1 a
Cold
Figure P1 b
Warm
Figure P1 c
Hot
Because adding heat energy usually results in a temperature rise, people
often confuse heat and temperature. In common speech, the two terms mean
the same: "I will heat it" means you will add heat; "I will
warm it up" means you will increase the temperature. No one usually
bothers to distinguish between these.
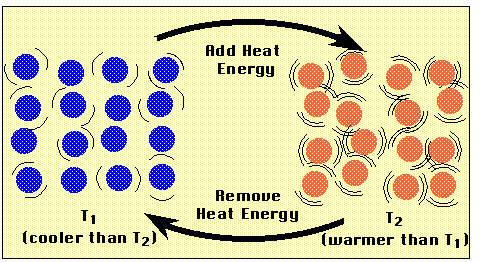
Figure P2a
Heat Transfer and Changing Temperature
Adding heat, however, does not always increase the temperature. For
instance, when water is boiling, adding heat does not increase its temperature.
This happens at the boiling temperature of every substance that can vaporize.
At the boiling temperature, adding heat energy converts the liquid into
a gas WITHOUT RAISING THE TEMPERATURE.
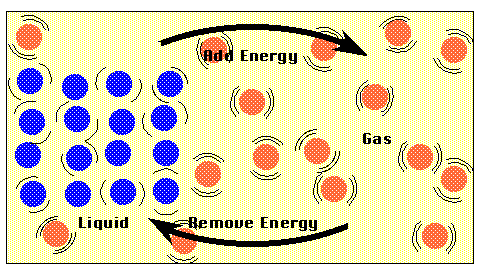
Figure P2b
Heat Transfer at Constant Temperature
Adding heat to a boiling liquid is an important exception to general
rule that more heat makes a higher temperature. When energy is added to
a liquid at the boiling temperature, its converts the liquid into a gas
at the same temperature. In this case, the energy added to the liquid goes
into breaking the bonds between the liquid molecules without causing the
temperature to change. The same thing happens when a solid changes into
liquid. For instance, ice and water can exist together at the melting temperature.
Adding heat to an ice-water slush will convert some of the ice to water
without changing the temperature. In general, whenever there is a change
of state, such as the solid-liquid or the liquid-gas transition, heat energy
can be added without a temperature change. The change of state requires
energy, so added energy goes into that instead of increasing the temperature.