Hands-On-Physics
Heat & Temperature
Messing Around:
HEAT FLOW
Your challenge is to detect the flow of heat energy. This is hard
because you don't have a detector for heat flow. You will have to infer
heat flow from what it does to temperature. The following list might give
you some clues.
- Heat energy flows away from anything that is hot toward anything that
is colder.
- Heat energy will flow until there is no temperature difference.
- The amount of heat energy flow depends on the temperature difference.
- You cannot measure heat energy flow directly, but you can infer it
by its effect.
Usually, heat loss will cool and heat gain will warm. So, if something heats
up, it is good bet that heat energy flowed into it; if it cools down, it
probably lost heat energy. It is this warming and cooling that you will
use to infer heat flow. If "A" cools, it must have lost energy.
If "B" warms it must have gained energy. If "A" and
"B" are similar, then they might even cool and warm similar amounts.
Remember, your challenge is to INFER the flow of heat energy. Do
this by measuring the temperature changes when there is energy flow between
two objects. For your experiment to be convincing, the heat energy leaving
"A" needs to be the heat that ends up in "B". You cannot
have extra loss from "A" or other sources of heat for "B".
Why is this important?
Set up your experiment:
Use a film can filled with hot water for "A". Use the same amount
of cold water for "B".
How can you arrange for all the heat "A" looses to get into "B"?
Hint 1
How can you be sure there is no other heat into "B"? Hint
2
Here is one way to measure the temperature inside the film can.
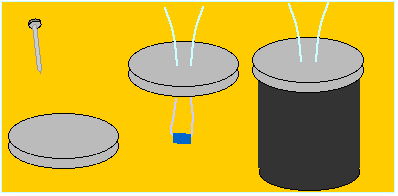
Figure M8
Film Can & Sensor
- Using a nail, make two holes in the top of a film can.
- Feed the leads of a temperature sensor through the holes and seal
with glue.
- Then, when the cap is on a can filled with water, you can measure
the temperature of the water.
If you are using a plain thermometer, you can do the same with just one
hole.
Now, to observe heat flow:
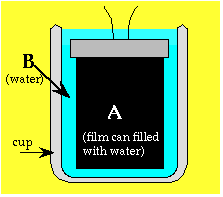
Figure M9
Heat Flow Apparatus
- Put hot water in the can (A).
- Put the can in the same volume of cold water (B).
- Put a second temperature sensor in the surrounding water.
- As soon as you place the hot can in the cold water, measure the temperatures
of A and B.
- When the temperatures stop changing, record both temperatures again.
How much did the temperature of A drop? How much did the temperature of
B increase in the same time?
- Repeat the experiment with different amounts of water in A and B.
How much did the temperature of B increase in the same time? What can you
conclude?
- Put crushed ice in A and then run the experiment. Explain your results.
What would you predict a graph of the temperature of A over time would look
like? Try it. Record temperature every 15 sec. Draw the graph. Put the temperature
of B on the same graph. What can you conclude?
Prepare a Report: Review the questions
, look at the suggestions for reporting,
and prepare a report on heat flow.
Hints
1. Put the film can inside a slightly larger
cup of cool water. All the heat leaving the film can will end up in the
surrounding water.
Use the same amount of water for both A and B. This means the can must fit
snugly in the cup. If necessary, you may need to fill up the part of the
cup with Styrofoam to take up some of the volume.
2. You need to reduce heat flow between the cup
and the room. Cover the cup with two layers of clear plastic to keep the
water from contact with room air. Hold the plastic on with a rubber band.
Use a Styrofoam cup to reduce heat flow through the sides. (Styrofoam cuts
down on heat flow; it is a bad thermal conductor.) If a Styrofoam cup of
the right size is not available, wrap your cup with several layers of dry
paper towels.
Previous
Page || Up a Level ||
Index || Next
Page


