Hands-On-Physics
Heat & Temperature
Messing Around:
GAS EXPANSION
Background:
Gas expands when it is heated and contracts when it cools. You can use this
to make a soda straw thermometer. If you trap some gas so that its pressure
doesn't change, then the volume of the gas will be proportional to the absolute
temperature. So, if you trap some gas but let it expand and contract, its
volume changes with temperature. You can use these volume changes to measure
temperature changes.
Construction Suggestions:
To construct a thermometer you can use a clear plastic soda straw.
Here is one way to do it:
Draw a plug of water into the end of the soda straw as shown below in figure
M10. Seal one end of the straw.
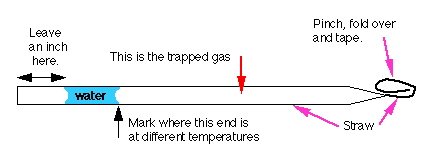
Figure M10
Soda Straw Thermometer
Measurements
- Place it in hot water, measure the temperature, and mark the straw
as illustrated.
- Repeat for cold water.
- Repeat for a few additional temperatures.
Questions:
- Where is the gas that is expanding and contracting with temperature?
- Why is one end of the soda straw left open?
- How accurate is the soda straw thermometer ?
- What are some of the problems of using this for general lab work?
- Using your data, what would be the temperature when the volume of
the trapped air would be zero?
Ideas for further investigation:
Try making a scale for your thermometer like those on commercial thermometers.
You could have a major division line every 20 °C.
The soda straw thermometer should be more sensitive by increasing the volume
of trapped air. Could you use a soda bottle? Build and test your design.
Prepare a Report: Review the questions
, look at the suggestions for reporting,
and prepare a report on your thermometer
Previous
Page || Up a Level ||
Index || Next
Page

